Membrane separation plays an increasingly important role in material concentration, sewage treatment and reuse of reclaimed water. Researchers strive to improve the permeability and anti-pollution property of water treatment membranes. There are many factors affecting the membrane performance, including surface roughness, charge, porosity, pore diameter distribution, hydrophilicity, etc. Among them, hydrophilicity is closely related to permeability flux of ultrafiltration membrane and anti-pollution property. The following is a theoretical explanation of how membrane hydrophilicity affects permeability and anti-pollution property.
PART 1: Effect of membrane hydrophilicity on permeability
Studies have shown that separation membranes from microfiltration to reverse osmosis applications should have good hydrophilicity to improve their wettability to achieve the highest water flux possible. Hydrophilicity is usually measured by contact Angle, which is shown in FIG. 1.If θ<90°, the solid surface is hydrophilic, that is, liquid is easier to wetting solid, the smaller the Angle, the better the wettability; If θ>90°, the solid surface is hydrophobic, that is, the liquid is not easy to moisten the solid, easy to move on the surface.
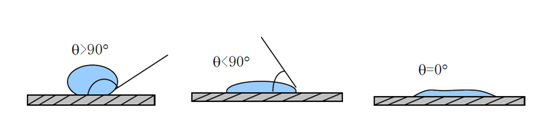
FIG. 1 Contact Angle of material surface
Many researchers have done a lot of work in improving membrane hydrophilicity. It is found that there is an intrinsic parameter of membrane material -- threshold pressure, and only when the transmembrane pressure difference exceeds this value can stable permeability be demonstrated. The threshold pressure of the hydrophilic membrane is close to zero and can be well wetted within the full pressure range, so the osmotic flux of the inner membrane in a certain range is proportional to the pressure. The relationship between membrane surface hydrophilicity and threshold pressure is shown in FIG. 2.
FIG. 2 Relationship between membrane surface hydrophilicity and threshold pressure
In contrast, membranes with strong hydrophobic pores have extremely high threshold pressure, and the wetting state is completely different under high pressure and low pressure, leading to great difference in permeability. Generally, hydrophobic ultrafiltration membrane has no flux in the dry state, so hydrophilic liquid is needed to dredge out the water producing channel, infiltrate the pore wall, and guide the membrane hole to produce water. Such hydrophilic liquid usually USES ethanol, isopropyl alcohol and other substances. Currently, due to the limitation of process factors, the membrane filaments prepared by general TIPS method are usually hydrophobic membrane filaments, and wetting agents are required before use to produce water. Once the membrane fibers are dry after leaving water during use, wetting agent is needed for re-use, otherwise it cannot produce water normally.
PART 2 Effect of membrane hydrophilicity on permeability on anti-pollution
During the use of ultrafiltration membrane, the flux will gradually decrease, although after cleaning, it cannot be restored to the initial state, which is due to membrane polluted. Membrane pollution refers to the treatment of materials in the particles, colloidal particles or solute macromolecules, due to physical and chemical interaction with the membrane or mechanical action, in the membrane surface or membrane pores adsorption, deposition, resulting in a smaller pore size or blockage, membrane separation performance degradation phenomenon. The pollution of ultrafiltration membrane is mainly divided into two types: one is adhesion, which includes the accumulation of suspended substances in the material liquid on the membrane surface (filter layer), the adhesion of organic substances on the membrane surface after concentration (gel layer), and the adsorption of colloidal substances or microorganisms on the membrane surface (adsorption layer).The other is blockage, that is, by the solute concentration, crystallization or precipitation in the material liquid to make the membrane fiber hole and produce different degrees of blockage.
By understanding the membrane pollution reason, study researchers both in China and abroad mainly from two aspects, to mitigate membrane fouling: on the one hand, in order to reduce the membrane surface in macromolecular solute precipitation amount as a starting point, namely reduce the phenomenon of concentration polarization of membrane surface material, fluid flow by changing the membrane surface, increase fluid turbulence level near the membrane surface, achieve the goal of reducing membrane fouling; On the other hand, starting from reducing the interaction force between the membrane surface and solute molecules, new membrane materials or modification of existing membranes and membrane materials can be used to increase the membrane's repulsion force against solute molecules and reduce its attraction to solute molecules, so as to achieve the purpose of inhibiting membrane pollution. The former can only alleviate membrane pollution to a certain extent, but cannot fundamentally inhibit membrane pollution; The starting point of the latter is to reduce the interaction between the membrane surface and solute molecules, which provides the possibility to fundamentally inhibit membrane contamination.
The hydrophilic modification of ultrafiltration, is to reduce the membrane surface and a method of solute molecular inter-atomic forces, mainly from the following four aspects: (1)the hydrophilic membrane surface covered with a large number of water molecules, when some water pollutants tried to do surface movement, must be "rejected" by water molecules, greatly reduce the chance that pollutants reach the surface of the membrane, restrain its adsorption;(2) Some hydrophilic groups of hydrophilic agents have a certain volume of rejection. When the pollutant is attracted to the material by the molecular layer on the surface of the material, the hydrophilic chain end is compressed, resulting in reduced conformation entropy and in an unstable state. (3)Hydrophilic water solubility makes the interface between the material and the pollutant liquid disappear, so as to eliminate the adsorption of pollutants on the surface of the material;(4) Hydrophilic polymer brush internal has a higher osmotic pressure, can resist the impact of foreign pollutants, to prevent the adsorption of pollutants in the membrane material surface.
PART 3 E-MEM ultrafiltration membrane hydrophilicity
E-MEM ultrafiltration membrane adopts the patented hydrophilic modification technology and amphiphilic copolymer modification. The membrane fiber has excellent hydrophilicity, high flux and good pollution resistance. The contact Angle of E-MEM membrane ultrafiltration membrane fiber is 60.4±3°, so it has a very low threshold pressure and is easy to be infiltrated by water. Compared with other brands in the same pollution condition, as shown in FIG. 3, the flux of E-MEM membrane ultrafiltration membrane is reduced, but still large. After simple backwash cleaning, the flux can basically return to the initial level, indicating that E-MEM UF membrane has excellent anti-pollution capability.
FIG. 3 Performance comparison of E-MEM ultrafiltration membrane fiber with other brands of ultrafiltration membrane
FIG. 4 Image of E-MEM ultrafiltration membrane filament


